Introduction
Software
Applications
Detectors
GM-10
GM-45
GM-90
Accessories
Test Source
Battery Box
Coincidence Box
Anti-Coincidence
GMI Interface
Air Sampler
Download
Linux
Users Group
Interfacing
Raspberry Pi
Random Numbers
Safety Uses
Radiation Info
Specifications
FAQ
Articles
Experiments
Rad Map
Links
Secure Order
Detectors
Contact
Radioactive Dust - Decay of Radon daughter products
Did you know that the dust that's in the air and settling all over your house (and computer monitor) is radioactive? It's true, it contains radioactive decay products from naturally occuring Uranium and Thorium.As an experiment, I wiped some dust from the TV screen onto a tissue, and placed it in front of the radiation detector. The reading went from a background reading around 10 CPM to around 1300 CPM, or 130 times the reading!
This graph shows the radiation (in Counts Per Minute, CPM) over time, as the daughter products decay. In addition to plotting the raw data, I've also estimated the initial amounts of Radium B (Pb214), Radium C (Bi214) and Pb212:
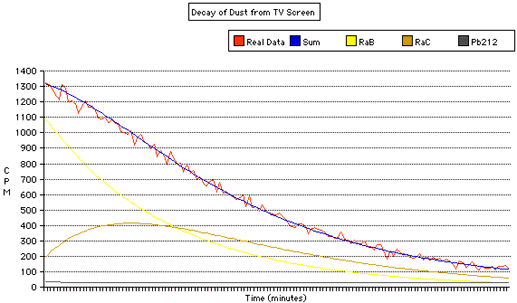
Radium B and Radium C are decay products of Radon. The Radon is produced by Uranium that naturally occurs in the soil, after a rather long decay process. Radon has a half life of just under 4 days. In addition to being produced by local sources, Radon (and it's daughter products) can be blown in by the winds from distant locations. So just because you don't have a radon problem in your basement doesn't mean that you won't find radioactive dust in your house!
The Radon decays into Polonium, which then decays into Radium B, an isotope of Lead. This decays with a half life of about 27 minutes in Radium C, an isotope Bismuth. This decays with a half life of about 20 minutes into another Polonium isotope, which quickly (164 microseconds) decays into an isotope of Lead. The Lead decays with a very long half life of 22 years, so we don't get much radiation from it, or any further products.
The lead and bismuth isotopes both undergo beta decay, and it is their radiation we detect in this experiment. Notice that we initially start with much more Radium B than Radium C. The Radium B decays, producing more Radium C, initially at a rate faster than the Radium C is decaying. So The amount of Radium C starts to increase. Eventually, there is not enough Radium B decaying into Radium C, and the amount of Radium C starts to drop.
Here's the process:
- Radon (Rn222) does an alpha decay into Polonium (Po218) with a half life of 3.824 days.
- Polonium (Po218) does an alpha decay into Lead (Pb214) with a half life of 3.05 minutes.
- Lead (Pb214) does a beta decay into Bismuth (Bi214) with a half life of 26.8 minutes.
- Bismuth (Bi214) does a beta decay into Polonium (Po214) with a half life of 19.8 minutes
- Polonium (Po214) does an alpha decay into Lead (Pb210) with a half life of 164 microseconds.
- Lead (Pb210) does a beta decay into Bismuth (Bi210) with a half life of 22.3 years.
- Bismuth (Bi210) does a beta decay into Polonium (Po210) with a half life of 5.01 days.
- Polonium (Po210) does an alpha decay into Lead (Pb206) with a half life of 138.38 days.
- Lead (Pb206) is stable.
The black line at the bottom of the graph shows the amount of Lead-212 present. This is a daughter product of Thoron, an isotope of Radon which is produced by Thorium, rather than Uranium. Thoron has a very short half life, about 1 minute. Lead-212 has a much longer half life, almost 11 hours.